Chemestry 11 Lessons Lewis Diagrams
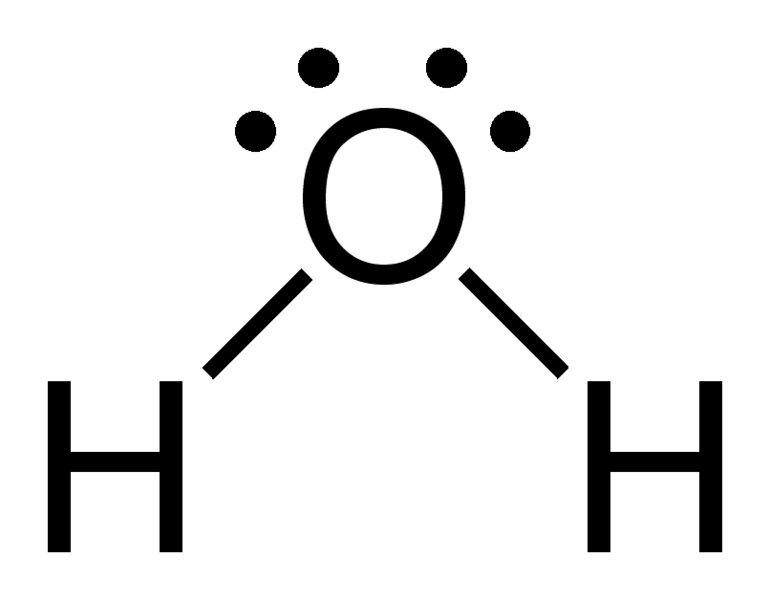
The Lewis Structure for water is useful because it allows to determine the molecular geometry and the polarity of the molecule. Because of the two lone pairs, H 2 O will have a bent molecular geometry and it will be a polar molecule. Remember that Hydrogen only needs two electrons to have a full outer shell. Video: Drawing the Lewis Structure.

Draw The Lewis Structure Of H2o Fotodtp
H 2 O lewis structure In the lewis structure of H 2 O, there are two single bonds around oxygen atom. Hydrogen atoms are joint to oxygen atom through single bonds. Also, there are two lone pairs on oxygen atom. Water molecule is a simple molecule. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions.

H2O Lewis structure and Molecular Geometry [No1 Best Explanation] Science Education and Tutorials
Step #1: Calculate the total number of valence electrons. Here, the given molecule is H2O (water). In order to draw the lewis structure of H2O, first of all you have to find the total number of valence electrons present in the H2O molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
H2O Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Lewis Structure of H2O Water, one of the Earth's major constituents, has the molecular formula H 2 O. A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound.

【4 Steps】H2O Lewis StructureLewis Structure for H2O (Water)Lewis Dot Structure for Water(H2O)
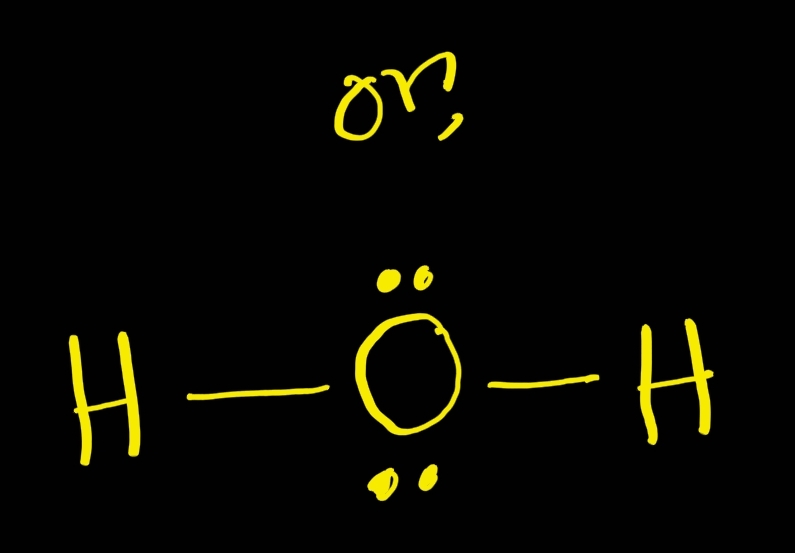
The Lewis structure is a representation of the valence electrons in an atom or molecule. For the H2O molecule, the Lewis structure shows that there are two hydrogen atoms bonded to one oxygen atom. Each hydrogen atom shares one electron with the oxygen atom, forming a single covalent bond.

【4 Steps】H2O Lewis StructureLewis Structure for H2O (Water)Lewis Dot Structure for Water(H2O)
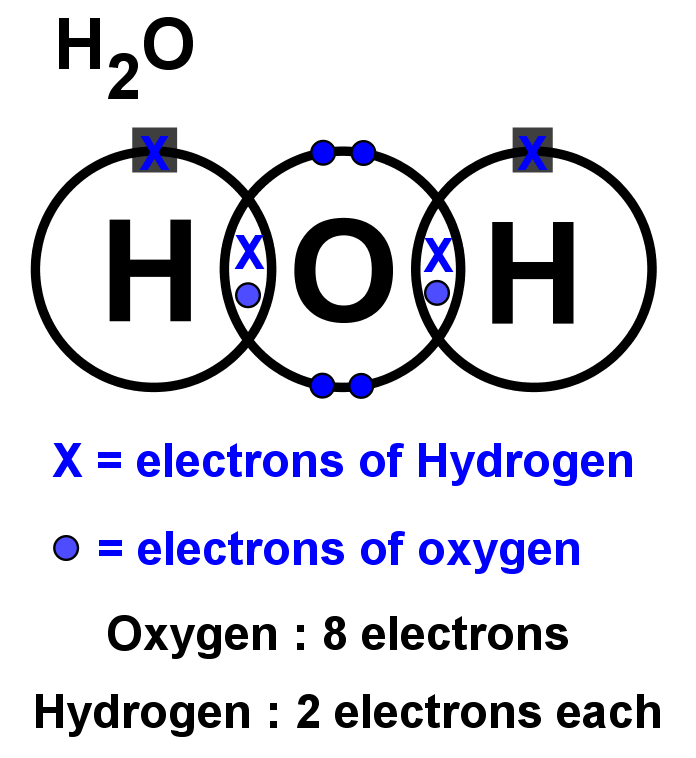
The Lewis structure of hydrogen and 2 oxygen atoms shows a total of eight valence electrons participate in the bond formation to form a single triatomic H2O molecule. Here, we need to understand how the Lewis structure is drawn for the H2O molecule: Look for the total valence electrons: It is eight to form a single H2O molecule.

H2O Lewis StructureHow to draw H2O Lewis Structure (Water)H2O Electron Dot Structure for
In the Lewis structure of H2O, the oxygen atom has 6 valence electrons from step 1, plus the 6 non-bonding electrons from step 4, giving it a total of 12 electrons. Since oxygen can accommodate a maximum of 8 valence electrons, we need to move a lone pair of electrons from the oxygen atom to form a double bond between the oxygen and hydrogen atoms.

H2O Lewis structure and Molecular Geometry [No1 Best Explanation] Science Education and Tutorials
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen.
Draw Step By Step The Lewis Structure For Water (H2O)
So starting off by drawing the Lewis structure: H 2 O: Water has four electron groups so it falls under tetrahedral for the electron-group geometry. The four electron groups are the 2 single bonds to Hydrogen and the 2 lone pairs of Oxygen. Since water has two lone pairs it's molecular shape is bent. According to the VSEPR theory, the electrons.

In this video we are going to learn about the Lewis structure of H2O. It is a chemical formula
A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc.

H2O Lewis structure and Molecular Geometry [No1 Best Explanation] Science Education and Tutorials
Here we will first place the atoms and individual valence electrons to understand the Lewis structure of H2O step-by-step. Oxygen atoms will take a central position as Hydrogen atoms always go on the outside. So place Oxygen in the center with both the Hydrogen atoms on the side.
Lewis structures StudyPug
1) Elementary models: The Lewis structure predicts that two lone pairs are (a) localized on the oxygen atom of water and that (b) both lone pairs are equivalent. The Lewis structure, combined with Valence Bond Theory, would predict that lone pairs occupy two equivalent hybridized \(sp^3\) atomic orbitals on oxygen.

H2O Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
First, we need to draw the Lewis structure of H 2 O. In short, these are the steps you need to follow for drawing a Lewis structure: 1. Write the correct skeletal structure for the molecule. * Hydrogen atoms are always terminal (only one bond) * Put more electronegative elements in terminal positions 2. Sum the valence electrons from all the atoms.

Lewis Structure Water Molecule Vector Illustration Stock Vector (Royalty Free) 2215045509
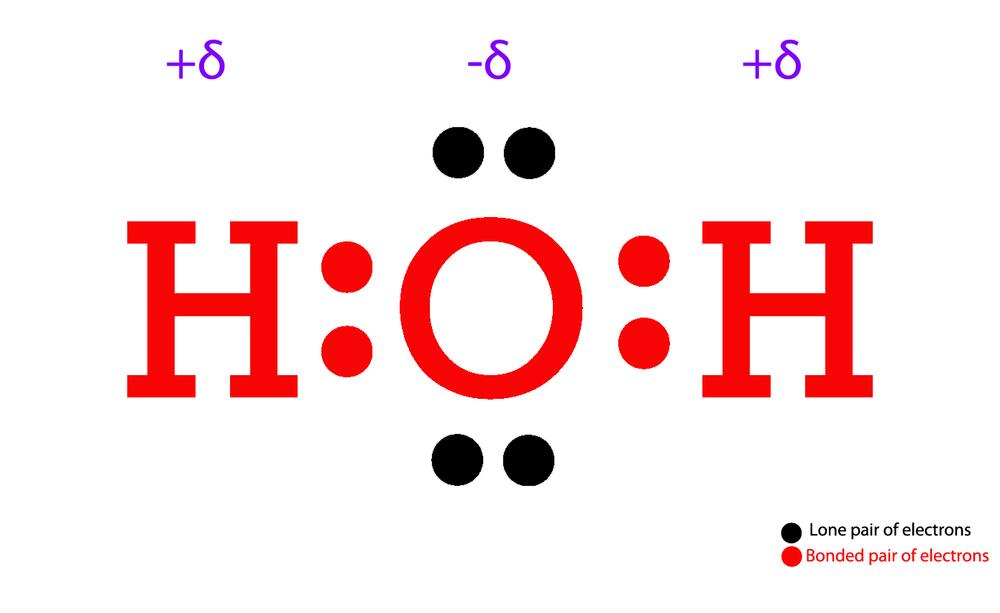
The Lewis structure of H₂O is Answer link You can find a procedure for drawing Lewis structures at this location. For H₂O, O must be the central atom The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell.
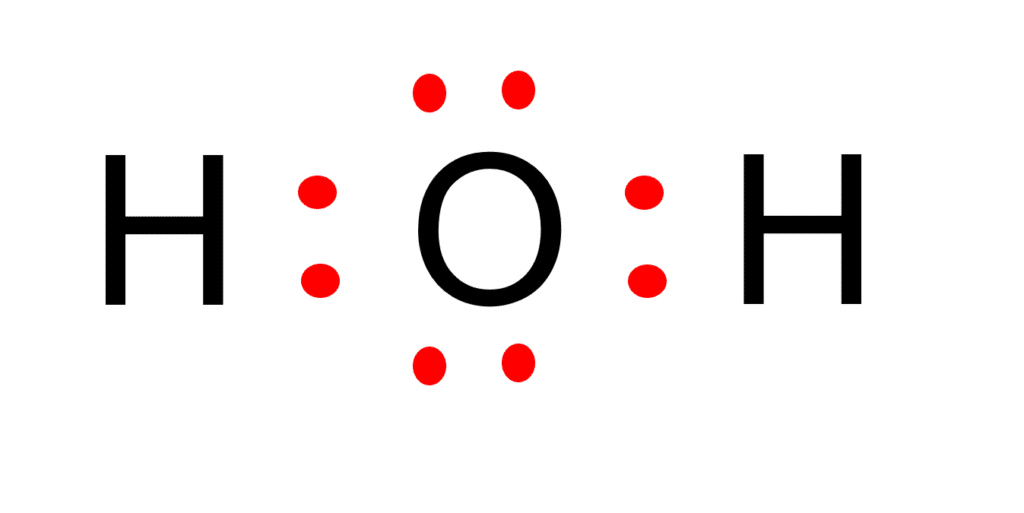
Lewis Structures Hydrogen (H2), and Water (H2O) What's Insight
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen.

H2O Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc.